3. The physiological properties of skeletal muscle fibres
3.1. Types of skeletal muscle fibres in terms of metabolism and functioning
L-A. Ranvier, the French anatomist who discovered the nodes of the myelin sheath wrapping the axons of neurons, described in 1873 how some muscles of the rabbit were redder, while other muscles were paler (Zierath, Hawley 2004), and this phenomenon was later observed in other animals. This characterisation also formed the basis for the typology of red–white muscle fibres. In the following, this subdivision has been refined.
In the slow twitch muscle fibres (these can be identified with the red fibres) there are many mitochondria, and therefore they are capable of carrying out a high performance oxidative energy production, that is, they do their job mostly in the aerobic work range. They have a relatively high quantity of the oxygen-storing protein called myoglobin which is structurally related to haemoglobin, and which helps cells out with oxygen in periods of temporary oxygen deficit. Their glycogen content is small, and so they are dependent on the nutrient content of the blood – the vascularisation of slow fibres is rich. Otherwise, these cells are given the name of red fibres because of the high concentration of myoglobin. Their most apposite name would be slow-aerobic fibres, but to help us remember them better, we may also call them red-slow fibres.
One type of the fast twitch fibres – the fast–anaerobic cell type – contains few mitochondria and little myoglobin, but they have a lot of glycogen. These fibres gain ATP fundamentally from glycolysis, so they mostly work in the anaerobic zone (but of course they also perform aerobic oxidation). These fibres have been described as white muscle fibres, and we can follow this denomination too.
The third type can also be regarded as an admixture of the previous two, because it contains the molecules and cellular components mentioned in intermediate quantities, its twitch is fast, but its metabolism is rather oxidative. These are the fast-aerobic fibres. To simplify, we could also call them red-fast fibres, but we should not forget that these fibres, compared to the classically red (red-slow, i.e. slow-aerobic) and the white fibres, have intermediate values in terms of the principal characteristics of their cellular components. The fundamental differences between the three muscle fibre types are summarised in Table 1 (Herbison et al. 1982; Zierath, Hawley 2004, Egan, Zierath 2013).
(According to another usual naming system, the muscle fibres may are also be categorised as type I, type II.A and type II.X [once called II.B]. Type I denotes the slow twitch, type II.A the fast, oxidative, and type II.X the fast, glycolytic fibres. Using our former denominations, type I corresponds to the slow-aerobic [red-slow], type II.A to the fast-aerobic [red-fast], and type II.X to the fast-anaerobic [white] fibres. A finer subdivision than this can also be made on the basis of diverse molecular characteristics [I, I.C, II.A, II.B, II.C, II.AB, II.AC; e.g. Scott et al. 2001], but the application of these categories would not provide any real benefit to us.)
We can emphasize the fact that the slow fibres operating mostly in the aerobic range, also play an important role in taking up the lactic acid produced by fast, glycolytic fibres, and utilising it with its oxidation. By doing so, they significantly contribute to the capacity of the aerobic system to refill the draining reserves of the anaerobic system.
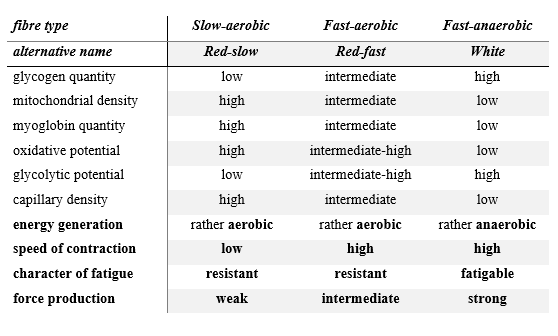
Table 1: The fundamental functional and metabolic differences between slow and fast twitch skeletal muscle fibres (Herbison et al. 1982; Zierath, Hawley 2004; Egan, Zierath 2013))
3.2. Muscle fibres at work
A motor neuron and the ensemble of fibres it innervates constitute a motor unit. A motor unit is characterised by one type of functioning, so it is composed of very similar fibres (Scott et al. 2001). On the basis of their contraction speed, we can find various units among the motor units: slow, fast-twitch fatigue-resistant, fast-twitch fatigue-intermediate, and fast-twitch fatigable units (Scott et al. 2001). Unlike the muscles described in rats, mice, guinea pigs, chickens, etc., which can be unambiguously classified as red or white types, human muscles are not formed of uniform motor units, but from this point of view, our muscles are mixed (Johnson et al. 1973; Herbison et al. 1982, Zierath, Hawley 2004): they always contain both fast and slow units, but in different proportions. We mainly find slow fibres in muscles requiring strenuous work, like for example in the antigravitational muscles responsible for posture; in contrast, a predominance of fast fibres can be observed in our fast moving limb muscles (Johnson et al. 1973).
A further fundamental difference between slow and fast motor units is that the slow units are usually smaller than the fast motor units. We can remember from our biological studies that during movements, smaller motor units come into action first (given that the threshold of their motor neurons is lower), and the ever larger units only later (this is the Size Principle, or Henneman’s Size Principle, of motor unit recruitment). This means that the units of slow fibres generally latch on to the contraction sooner, and the larger units containing fast fibres come into play only during powerful, fast movements. A curiosity is that, because of this, two processes which have a muscle mass lessening effect, act on fast and slow fibres differently. When there is an immobilisation effect – such as when a part of the body is put in plaster – it is primarily slow fibres which suffer degeneration. An explanation for this may be that fast fibres are a priori “accustomed” to be excited more rarely, being inactive for most of their life, thus, the absence of stimulation rather affects slow fibres which receive an almost continuous stimulation in the course of our daily routine. However, the other effect, aging, causes a larger scale reduction in the quantity of the fast fibres, which can explain the more marked decrease in speed and force amongst the elderly (Radák 2016). For this reason, physical activity, moreover, strength training also are of high priority in older age.
The fibre type differences characterising the single muscles can also be observed between individuals, since the ratio of fibre types varies even in the same muscle within the population (e.g. Zierath, Hawley 2004). This explains why some demonstrate talent in endurance sports, whereas others do so in sprinting sports. It can be said, at the same time, that with tenacious training work, the fibre ratios in our muscles can – to a certain extent – also be changed (e.g. Scott et al. 2001; Handschin, Spiegelman 2008; Kelly 2012; Viollet 2018). As an effect of training, for example, in the leg muscles of long distance runners, the proportion of red muscle fibres increases, while in those of sprinters it decreases over the years.
Studies of the muscle composition of athletes doing different sports showed, 40 years ago, that the fibre composition in the muscles of athletes engaging in endurance and sprint sports differs from each other (e.g. Costill et al 1976). They reinforced the previously held view that the genetic properties of our muscles constitute one important element of success; nevertheless, these studies did not take into account the changes caused by sport. Fink and co-authors (Fink et al. 1977) compared the composition of the gastrocnemius muscle of elite (male) distance runners, good but not world class runners, and untrained men, and they found that the proportion of slow fibres in top athletes (79% ± 3.5%) was much greater compared both to that in good runners (61.8% ± 2.9%) and that in untrained individuals (57.7% ± 2.5%). However, on closer inspection of the data, it turned out that the different ratio of fibre types alone could not explain the differences observable between individual performances. For example, if we compare the data of two elite runners with very similar best marathon results, we can see that there is an enormous difference between them in terms of slow fibre ratio: 98% and 50% (Fink et al. 1977). Thus, we can conclude that the proportion of fibre types does not necessarily constitute a solid base for predicting performance, but other factors also need to be taken into account (Zierath, Hawley 2004). It is not insignificant, for instance, how much air an athlete can inhale at once, what stroke volume his/her heart can perform, the transport capacity of his/her vascular network which carries the oxygen to the tissues, how much oxygen the musculature can extract from the arriving blood, and – not least – the lactate tolerance of the body. In the following, we will review these limiting factors and their connections.