2. Production of energy in the human body
2.1 Where does energy come from?
In our life, the basis of every activity is the production of the appropriate quantity of energy needed to do it. Since sport is an activity with an elevated level of movement, the understanding of the process of energy production and a knowledge of its influencing factors play a central role in sports physiology. During the production of energy, in essence, we do nothing but make available the solar energy once enclosed in the chemical bonds of diverse molecules by the autotrophic living systems (mainly plants) residing at the beginning of the food chains.
Green plants produce the organic large molecules which represent the basis of life, from simple, inorganic materials (CO2, H2O) and luminous energy, meanwhile emitting oxygen into their environment as a by-product. The build-up of organic substances means carbohydrates (glucose; C6H12O6) in the first place, and then, with the various modifications of these, they generate lipids (fats and oils), proteins and nucleotides. We may remember from our biological studies that the backbone of all organic substances (“biomolecules”) is made up of a carbon skeleton formed by carbon atoms attaching to each other, and, depending on the type of the given material, the atoms of hydrogen, oxygen, nitrogen, phosphorus, and sulphur (and other atoms and ions in a much lesser proportion compared to the previous six) are attached to this skeleton.
The process of photosynthesis, therefore, simply (and without showing the molar quantities) looks like this:

So plants (and other autotrophic creatures) generate the larger molecules needed by living systems. These are taken up by us through food, and broken down into their smaller units (building blocks) during digestion. These smaller units, such as
glucose deriving from the demolition of carbohydrates,
fatty acids and glycerine, which form lipids,
amino acids that are the products of protein degradation, and
the diverse derivatives of nucleotides
can, on the one hand, be used to build up our own substances (our own proteins, carbohydrates, lipids and nucleotides). On the other hand, those not utilised as building materials are further disassembled, until we get carbon dioxide and water again, and, most importantly, energy. This process is cellular respiration or biological oxidation.
Thus, during respiration, in broad terms, the following process takes place:
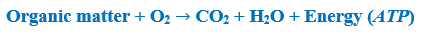
Note that the processes of photosynthesis and respiration are exactly the opposite of each other. The only difference is the direction, and that the “energy” member has a different quality (in terms of quantity, they are precisely equivalent). So, during respiration, we break down the organic matter (even the carbon skeleton) which had once been produced by the autotrophs (although it is possible that it was taken up by the body of another heterotroph animal). To do all this, we use the oxygen that had been emitted by the autotrophs into the environment during photosynthesis. (The oxygen will be transformed into water, as it had been previously, and the carbon skeleton will be transformed into carbon dioxide in the same form in which it had been taken up by autotrophs.) So from the whole process of cellular respiration it is important for us to liberate the energy stored in chemical bonds (energy which was once derived from solar energy), and to transform it into a usable and storable form. The molecule called adenosine triphosphate, ATP for short, serves this aim. ATP is a simple nucleotide that stores a great quantity of energy in the bonds between its three phosphate groups (PO43-). Ceding one of the phosphate groups it – reversibly – transforms into adenosine diphosphate (ADP), and in the meantime, it provides energy for a linked reaction (Figure 1).
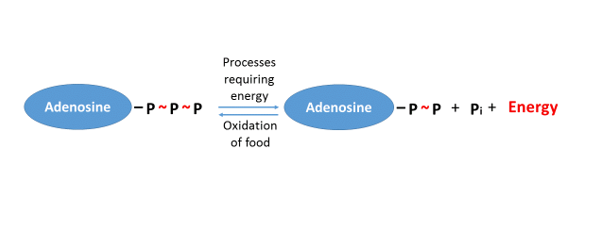
Figure 1. Schematic representation of the transformation ATP–ADP
(For convenience, the phosphate group is denoted by the letter P, while Pi stands for inorganic phosphate)
Thus, the energy which is released in the process ATP → ADP + Pi is used by another reaction. In our cells, a great many types of energy-requiring reactions take place, but in the other pan of the scales, we always find that the ATP molecule is broken down. This can also be understood by imagining the ATP in the human body as a universal “currency”, since it is this molecule which provides the energy for all the energy-requiring biochemical transformations. This, however, is not only valid for the human body, but also for the whole living world: from bacteria to mammals, almost every energy-requiring metabolic process is paid for by ATP. The key role and the ubiquity of the molecule is clearly shown by the fact that during our routine activities, the quantity of ATP transformed each day is roughly equal to our body mass (e.g. Törnroth-Horsefield, Neutze 2008). So, the many different types of reaction deplete ATP, and therefore the consumption of ATP is one side of the coin. On the other side, there is the regeneration of ATP. This mainly occurs in the cell organelles called mitochondria. The mitochondrion contains the molecules and reaction conditions which make it possible for the inorganic phosphate group (Pi) to rebind to the ADP. The contraction of our muscle cells also requires the breakdown of ATP: we may remember that myosin is an APTase enzyme. So it is no accident that the alpha and omega of sports activity is to produce a proper quantity of ATP in a given time. In the following, we overview how our body produces ATP.
2.2 The breakdown of carbohydrates: cellular respiration
As we have seen, the production of energy always starts with the breakdown of organic matter, and this requires oxygen (see the gross equation of respiration). The most important food for our cells, in the sense of “fuel”, is the six-carbon glucose. With the help of cytoplasmic enzymes, in several steps it is split into two three-carbon units, known as pyruvic acid (the ion of which in aqueous solutions is pyruvate). The process is called glycolysis. During this process, 2 moles of ATP are generated from 1 mole of glucose.
Glycolysis:

The pyruvic acid then enters the mitochondrion, and its final degradation will start. In the course of this process, it first loses a carbon atom and transforms into an acetyl group (C2). This is important because the breakdown of all other types of organic matter (lipids, proteins and nucleotides) finally converges into the two-carbon acetyl group.
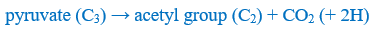
The acetyl group then enters a cyclic process called the citric acid cycle, in which it attaches to a four-carbon compound which, in this manner, turns into a six-carbon molecule. Whilst it recombines to the original four-carbon molecule in several steps, it first loses one, and then another, carbon atom. Thus, in the long run, in the citric acid cycle, the bond between the carbon atoms of the acetyl group breaks, and 2 (moles of) CO2 molecules are produced. These are transported into the lungs by the blood, and exhaled from there; thus, they are given back to the environment in the form in which they were taken up – and may also be taken up again – by plants.
The closing chord of the process is terminal oxidation. In this stage, the hydrogen which has been liberated during the citric acid cycle and the previous reactions is broken down into electrons and protons. The electrons are put on a chain consisting of molecules whose reactions are linked one after the other (the electron transport chain or respiratory chain), and finally, they are taken up by the oxygen diffusing from the blood to the cells. The oxygen also takes up the protons along with the electrons, and hence it is oxidised to water, which occurs together with the liberation of a great amount of energy. In essence, it is this energy that is used to regenerate ATP (ATP-synthase enzyme: ADP + P → ATP), whilst a part of the energy – more than half of it – turns into heat. From the energetic point of view, this heat represents a loss, but in cold weather, from the point of view of heat generation, it is a gain. (In the living world, in terms of energy generation [ATP production], oxygen is the universal acceptor molecule of the electron, but other solutions exist, too: some bacteria – for example, those living in submarine hot springs – use sulphate or nitrate as the electron acceptor for their anaerobic respiration.)
To sum up, with the total combustion (oxidation) of one mole of glucose, altogether 38 moles of ATP are generated, which, however, is only a theoretical value, not the value obtained by the cell, because certain processes of the oxidation use up some of this quantity in the meantime. Understanding this process makes it clear why it is so important to provide oxygen to our muscle cells: for movement, muscles have to contract, for this they need ATP, for the generation of which, oxygen is needed. The longer we are able to supply the necessary quantity of oxygen and the fuel substances of oxidation, the longer we are able to generate ATP. If enough oxygen is available in our muscle cells in the course of the activity, then we are using the muscles in aerobic conditions.
2.3 An alternative route: fermentation
The most important condition of the breakdown process described above, besides the different enzymes and starting substances, is thus the presence of oxygen. If there is not enough oxygen in the mitochondria, then there is nothing to accept the electrons at the very end of the process. In this way, the electron transport chain is partially “engorged”, and the system is no longer capable of satisfying the ever growing need for ATP. Activity occurring in a relative absence of oxygen is called anaerobic activity. The body, however, during strenuous activity, after a while needs more ATP than can be produced with the oxygen we are able to take up. Fortunately, the production of energy does not stop: in the absence of enough oxygen, a proportion of the pyruvic acid produced during glycolysis does not go on the metabolic pathway towards terminal oxidation, but towards the process called fermentation. Fermentation is an energy producing process of low efficiency, because, compared to full oxidation, it only generates approximately 1/15 the amount of ATP. There are two main types of fermentation in the living world, depending on what product the pyruvic acid will turn into. In some living systems, for example in yeast, the product is ethyl alcohol (C2) + CO2. This is called alcoholic fermentation. In the human skeletal muscle cells the product is lactic acid, and its ion, forming in an aqueous solution, is called lactate. In essence, during fermentation, the pyruvate, generated in the glycolysis, is converted into a product (to lactate acid) which can easily be transported away from the cell, and takes up the H atoms liberated in the glycolysis. This is necessary because if a product of a reaction piles up excessively, this stops the reaction. Therefore, if the pyruvate is converted and transported away, it no longer inhibits the production of further pyruvate molecules, and the molecules which take up H atoms can also queue up for further hydrogen atoms during the glycolytic scission of the following glucose molecule. During fermentation, we may only calculate a gain of 2 ATPs per glucose, those that have been generated during glycolysis.
Thus, the utility of fermentation is that
the production of energy can go on even without satisfying the extended demand for oxygen, and that
it occurs more rapidly than full oxidation.
These properties make it possible for the muscles to use fermentation – in addition to cellular respiration – as an accessory ATP-producing source during forceful activity. However, fermentation has its price, because the lactic acid produced only offers a temporary solution for the oxygen-poor period, while from the energetic point of view, it generates a “debt” (see also later). Besides this, lactic acid in a too high concentration endangers the functioning of the body, so our cells have to do something with it. In the following unit, we overview how the cells and the body regenerate from the products of fermentation.
2.4 The Cori cycle
The lactic acid produced in the muscle cells can be reconverted to glucose by the liver. The entire process is of cyclical character: the lactic acid is pumped from the muscle into the blood which carries it to the liver. The enzymes found here make glucose from the lactic acid again in a few steps (gluconeogenesis); this goes into the blood again, from where it can be taken up again by the muscles which can transform it again into lactic acid during their anaerobic energy production (Figure 2).
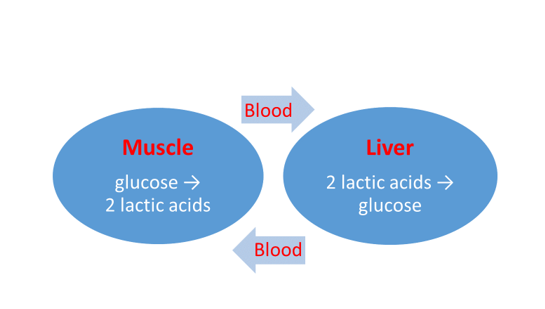
Figure 2. The Cori cycle
The importance of the Cori cycle is that it
- eliminates lactic acid which has attained a too high concentration, from the muscle cells involved, and
- provides reusable food to the muscles.
As has been mentioned, in terms of energetics, however, fermentation produces an overall debt. The liver can accomplish the transformation into glucose at the expense of 6 ATPs, that is, subtracting the gain of 2 ATPs of the glycolysis, lactic acid fermentation produces a loss of 4 molecules of ATP per glucose. Thus, the cycle cannot go on endlessly. Of course, this amount of ATP has to be produced later, with the help of mitochondrial terminal oxidation, which thus requires the expenditure of extra oxygen from the body. So, in effect, the Cori cycle temporarily passes the cost of the high intensity working of muscles to the liver, but in the long run, the body has to pay back the created debt in the period of lowered performance (i.e. of reduced ATP need). Hence, the process is not a panacea, but only helps the body survive the period during which the energy demand is too high in the given muscles.
2.5 Lactic acid as fuel
Besides the Cori cycle, skeletal muscles are also capable of reconverting lactic acid into pyruvic acid, and then totally disassembling it into carbon dioxide and water in the mitochondrion, while they generate ATP during the aerobic cellular respiration (e.g. Bangsbo et al. 1995, Garnier et al. 1996). So, from this point of view, the lactic acid only provides a temporary “storage place” for the hydrogen atoms produced in the glycolysis. These hydrogen atoms, however, sooner or later, have to return to their “place of destination”; that is, they have to meet oxygen during the aerobic pathway of energy production, forming water (Figure 3). A question may then arise: how does the muscle accomplish this aerobic oxidation, given that it is precisely at the point when it is producing lactic acid that it is unable to achieve a sufficient level of aerobic energy production, because there is not enough oxygen in it? Naturally, the muscle does not combust lactic acid in this period, but only when work intensity drops – or alternatively, if the muscles performing the combustion are not the ones which had produced the lactic acid, but those that still have the capacity for aerobic ATP production, because they do not work at high intensity.
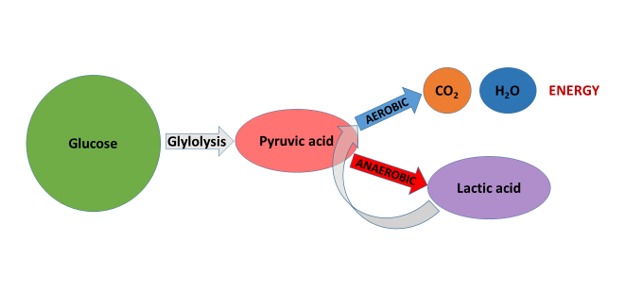
Figure 3: In muscles, lactic acid, generated in anaerobic fermentation, sooner or later enters the process of aerobic energy production
2.6 The power sources of muscles in terms of stored substances
2.6.1 Muscles’ own energetic reserve: creatine phosphate
Muscle cells also have a direct ATP reserve which refills the ATP consumed, for a brief period. This is the molecule called creatine phosphate (or phosphocreatine), and it regenerates the ADP formed, by transferring its phosphate group:

In this way, muscle cells can begin a new contraction. Then, with the help of the ATP forming in parallel with, or after, this process (in the glycolysis and the terminal oxidation), the molecule is reconverted into creatine phosphate, as a reversal of the previous process (Figure 4). This reservoir in the muscles is sufficient to cover the ATP demand for a mere 10-15 seconds of maximum intensity work (e.g. Fonyó 2014). So, in terms of power output, this chemical reserve represents a very important accessory mechanism which is much faster, but also much more rapidly exhausted, than fermentation.
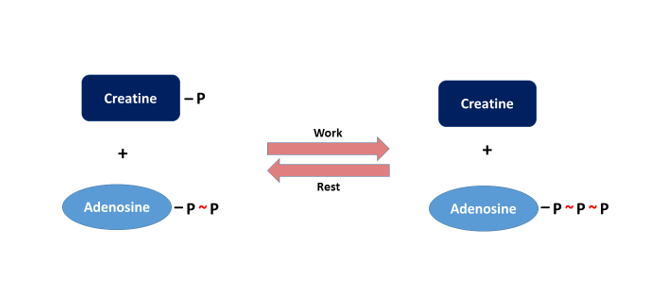
Figure 4: During contraction, an enzyme (creatine kinase) can convert ADP into ATP with the help of the phosphate group of creatine phosphate, while during rest, at the expense of excess ATP, it creates creatine phosphate from creatin
2.6.2 Fuel utilisation and the muscle’s own fuel reserves
It is a characteristic of the utilisation of fuels in the muscle that they oxidise predominately two types of organic matter, both at rest and under load: carbohydrates and fats (fatty acids are broken down into acetyl groups in the same way as pyruvic acid, which is generated from glucose, and then they are fully oxidised to carbon dioxide and water, and ATP is generated in the meantime). However, the ratio of the utilisation of these substances differs, depending on the intensity and duration of training. The fatty acid supply of muscles is mainly covered by the breakdown of the fat of fatty tissue (its mass is variable, cca. 5–40 kg), but the muscular tissue itself also has a smaller (300 g) fat reserve (intramuscular fat) which begins to be broken down during training (Jeukendrup 2003, Kiens, Hawley 2011). This is important mostly during training sessions of medium intensity and long duration (more than 90 minutes), when it provides a quarter of all energy consumption. At lower or higher intensity, the importance of intramuscular fat in energy provision decreases (Egan, Zierath 2013).
Besides this, muscle cells also possess a significant glycogen reserve (approximately 50–900 g in total). The giant molecule called glycogen is a polymer constituted by glucose units, which ensures glucose for energy production (by means of enzymatic demolition) when needed. During sports activity, the glycogen content of muscle cells also begins to be broken down, and the glucose produced yields energy by entering the process of full oxidation or that of fermentation. The glycogen needs of the muscle, besides its own reserves, are covered by the glucose which is derived from the breakdown of the liver glycogen (80–100 g), and taken up from the blood. On the whole, fat reserves represent 92-98 %, while carbohydrate reserves only make up 2-8 %, of the energy reserve of the body (Jeukendrup 2003).
If work lasts longer (over several hours), the muscle’s own, local reserves become progressively depleted. After this, the muscle is dependent on external fuel reserves (i.e. those which come with the blood). These compounds are the glucose coming from the liver (and which, after the depletion of liver glycogen is created by gluconeogenesis), and the free fatty acids coming from the breakdown of the fats in fat tissue; what is more, the muscle can also oxidise ketone bodies. (Ketone bodies are formed in the liver, from the acetyl groups generated from the breakdown of fatty acids, especially during fasting but also during a considerable loading. The brain and the heart are able to use two of them – β-hydroxybutyrate and acetoacetate – as alternative combustibles instead of glucose. The third ketone body, the acetone forming from acetoacetate, leaves the body through the lungs: this is the “acetone breath”). However, the relative contribution of these substances to energy production will be different: under sustained sports activity, as times goes by, the extent of the utilisation of carbohydrates diminishes, while that of fatty acids increases (Jeukendrup 2003).
So long as the intensity of training is augmented, initially the extent of the use of both carbohydrates and fats increases, but after a certain limit, the utilisation ratio of fats gradually diminishes, while that of carbohydrates increases (Achten et al. 2002, Jeukendrup 2003, Kiens, Hawley 2011; see also later chapter 5.2). With the increase in intensity, the principal fuel will be muscle glycogen, and the use of this increases proportionally with the rise in intensity (Egan, Zierath 2013).
It must be noted that the body can also utilise proteins as sources of energy, but the oxidation of amino acids coming from the breakdown of proteins proportionally yields only a tiny quantity of energy. During training, the contribution to energy production made by amino acids is less than 1% (according to other authors, it is 2–5 % [Moore 2015] and 1–6 % [Tarnopolsky 2004]), and it is under 10 % even during sustained physical activity by a fasting body (Jeukendrup 2003).
2.7 The multi-faceted lactic acid
We have seen that lactic acid plays an important role during muscle work, but beyond this, it also performs many other functions in our body. Lactic acid, once only regarded as a by-product, has proven in recent years to be an important signalling molecule in the human body, playing a role, for example, in the attenuation of inflammation, in wound healing, in the regulation of energy balance, in memory formation and also in tumour progression (Sun et al. 2017).
During our daily activity, a quarter of the lactic acid going into the blood comes from muscles, a quarter from the brain, a quarter from the skin, and the rest arrives mainly from red blood cells and from the guts (Sun et al. 2017).
Of course, during physical activity, the lactic acid emission of muscles increases to a large extent, which is compensated for by the growing lactic acid uptake of the liver (which can experience as much as a tenfold increase [Sun et al. 2017]), since this organ is capable of reducing the excessive concentration of lactate via gluconeogenesis (to a lesser extent, the kidneys also fabricate glucose from lactate).
As we have seen, skeletal muscles which are not working at a high intensity behave in a similar manner, that is, they are operating in the aerobic mode to produce energy: they also take up the lactic produced by their peers, reconvert it into pyruvic acid, and then oxidise it in the mitochondria (e.g. Bangsbo et al. 1995, Garnier et al. 1996). In doing this they – as it were – take over from the debt expressed in ATP of muscles operating in the anaerobic zone. In addition, the heart muscle also takes up lactic acid, and utilises it as a combustible (in the same way as skeletal muscles). This is a significant energy source for the heart, given that cca. 10-15 % of its fuel supply needs are met by lactic acid at rest, and cca. 30 % during physical activity (Sun et al. 2017).
2.8 Summary of the theoretical foundations from the point of view of sport
In the light of the above, we can now summarise which metabolic factors fundamentally determine physical work, and thus sports. If the activity is not too intense, that is, enough oxygen is available, then energy production is mostly achieved by the oxidation of glucose and fatty acids (nominally yielding 38 molecules of ATP per glucose molecule). This process eats up the glycogen content and the modest fat reserve of the muscle at the beginning, and it continues with the combustion of the fat of fat tissue in the longer term. Having ‘enough oxygen’ more precisely means that our capacity to take up oxygen is proportionate to the required work intensity, that is, we are able to “cope with the effort with more air”. By doing sports, we can significantly improve this capacity (see later). In these conditions, a sports workout will mostly be done in the aerobic mode (Figure 5). So, in this working zone, two crucial points are the time factor and the quantity of fuel: how long we are capable of giving fuel to the muscles, how long we can ensure glycogen and fat, and in which period of the competition we must replace the draining reserves, for example, with beverages with a high nutrient content. This, typically, is the performance zone of marathon runners, triathletes, long distance swimmers, bicycle road racers and ironman competitors.
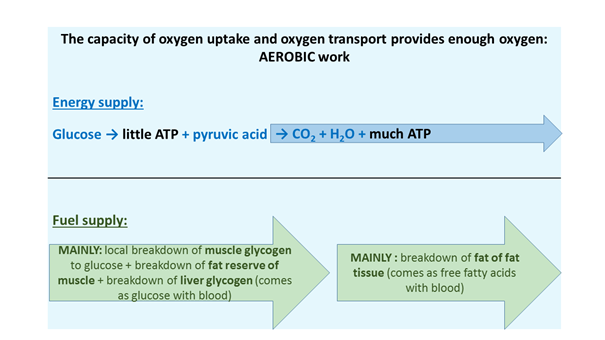
Figure 5: The aerobic supply of energy and fuel)
If, however, the activity is more intense than this, cellular respiration requires more oxygen than the blood can carry there and the cells can take up. So, the aerobic capacity is no longer able to provide the ATP production needed, and so another portion of energy generation has to switch to an anaerobic lactacid – i.e. anaerobic, lactate productive – working mode to an ever increasing extent. It is very important to highlight that, naturally, cellular respiration does not stop during this process, cells only have to find a solution to the problem of generating the extra ATP (Figure 6). For the initial few seconds, creatine phosphate can provide an adequate quantity of ATP, but in most sports – except for sprint-race sports – the extra ATP is required for a much longer period. In these instances, the pyruvic acid which has been formed in the glycolysis does not enter the mitochondrion but is converted into lactic acid (i.e. fermentation starts). This exits the cell, and is transported into the liver, in order to be reconverted into glucose during gluconeogenesis (Cori cycle). A further quantity of it goes into the heart, and what remains is taken up by the skeletal muscles which are not working at such a high intensity. As long as the lactate-glucose exchange – effectuated by the blood – between the liver and the muscles, plus the lactic acid absorption and combustion by the heart and the other skeletal muscles sufficiently counterbalance lactate production, this working intensity can be sustained. With a further intensifying of performance, the concentration of lactic acid generated by anaerobic energy production continuously increases in the blood. After this, the level of performance is determined by how high a blood lactate level the body can tolerate. This capacity – the extent of lactate tolerance – can also be developed.
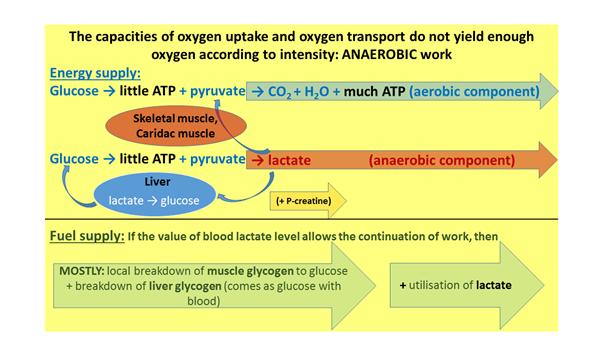
Figure 6: Anaerobic performance zones of muscle work in terms of oxygen and fuel supply.
The anaerobic work can be stopped by a too high blood lactate level in any phase of fuel utilisation. The smaller yellow arrow shows that the creatine phosphate, serving as an ATP reserve, can provide energy for a limited period, in phases of activity performed with great force. With respect to fuel utilisation, at high intensity the body fundamentally uses carbohydrates, and besides this, it also strives to harness the lactate produced
The third important component of the system is the phosphocreatine system. This yields ATP very quickly to satisfy the need encountered during increased performance, through fast chemical steps, but only for a very brief period. We call this system anaerobic alactacid, that is, an anaerobic system which does not produce lactic acid. The anaerobic alactacid energy production can be imagined as a sort of “turbocharger”, since besides the continuously ongoing aerobic ATP production and the ATP generation by the rapid lactate synthesis, it contributes to the system with an extra reserve which makes it possible, if only for a few seconds, to significantly boost the performance of our muscles. If this store becomes exhausted, then the great amount of ATP formed during terminal oxidation must replenish it. Therefore, this extra energy source can be switched on multiple times during sports activity, if we pay attention to also having more economical performance periods in the course of the activity, during which we can restore its ATP reserve.
Now, in many books and magazines concerned with sports physiology, the aerobic, anaerobic lactacid and the anaerobic alactacid energy producing modes are described as linear, sequential units (Figure 7. a). These texts often lead the reader to infer that during sports activity, the aerobic system works first, and then the anaerobic lactacid system follows. And the phosphocreatine system is mentioned as the reserve ensuring the energy provision for the initial, 10-15 second long period of an activity which immediately begins with an output of explosive performance. They do so despite the fact that a long time ago it was clearly described that in the skeletal muscles, both the lactacid and alactacid anaerobic systems are already functioning when the aerobic pathway is still largely capable of producing energy (e.g. Sahlin et al 1987). They also falsely suggest that during greater efforts, the anaerobic system takes over energy production from the aerobic one (Figure 7. b). If this were the case, the intensely working muscles would not need oxygen only after the intense work has stopped. Likewise, the creatine phosphate store is not only rechargeable after loading has ceased, during rest, since the extra energy provided by it cannot be deployed only once during an activity. These considerations are contrary to experience, and are also illogical.
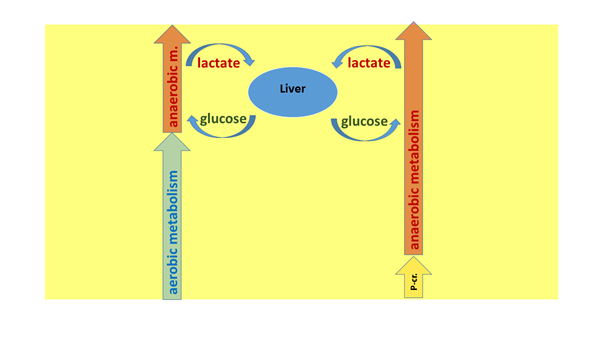
Figure 7. a: An ERRONEOUS model of the energy productive pathways.On the left side we can see the process scheme of the aerobic–lactate productive supposed functional sequences, and on the right side that of the creatine phosphate–lactate productive supposed functional sequences
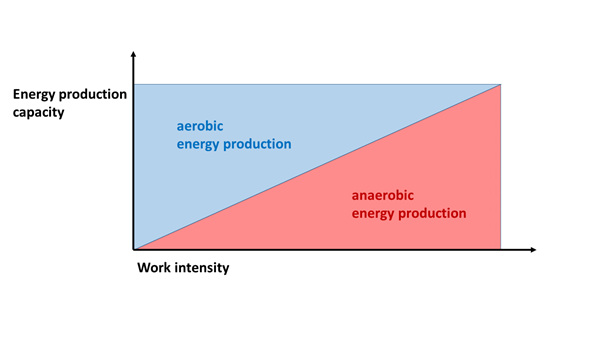
Figure 7. b: According to the ERRONEOUS model, with an increase in intensity, the anaerobic energy production gradually takes over the role of the aerobic metabolic pathway)
Based on the notions reviewed above, the contours of a much more realistic system are logically delineated (see, for example, Jamieson 2017). The three energy producing systems (aerobic, anaerobic lactacid and anaerobic alactacid) are metabolic pathways functioning side by side all the time; however, their relative significance differs. The basis of energy production is provided by the aerobic system. This is not only because the aerobic system has a large and economic capacity of generating ATP, but also because it is the only one that can repay the energy cost conveyed by the lactic acid produced in an ever growing quantity with increasing working intensity. The lactate generated by the anaerobic lactacid system is utilised either in the Cori cycle, in the myocardium (heart musculature), or in the skeletal muscles, but always in aerobic processes (the ATP cost of the gluconeogenesis of the liver, the oxidation going on in the heart muscle or in the skeletal muscle).
Thus, one limit to the boostability of performance is represented precisely by how long the variously acting aerobic processes are capable of covering the accessory costs of the fast ATP synthesis of the anaerobic lactacid pathway. That is to say, until that point, lactate does not accumulate to a dangerous extent in our body. If we go over this point with intensity, and we cannot restore the damage caused by the debt in the successive, less forceful work period (or else there is no less forceful period), then, at one point, the accumulating lactate gives a stop command to the activity. If, however, we insert more “relaxing” periods into the training/competition, then during these, we can regenerate the lactate-debt formed by the anaerobic lactacid system. When performance has to be boosted to an extreme extent, then our anaerobic alactacid system (i.e. the one provided by creatine phosphate) “cranks up” its work, and of course, this system can also be restored with the help of the aerobic system, in a period of lesser intensity. In sports practiced with variable intensity – e.g. in ball games and in combat sports – these are fundamental and frequent methods for boosting performance; in the linear, old model all this does not receive a satisfactory explanation. The connections described are illustrated in Figure 8.
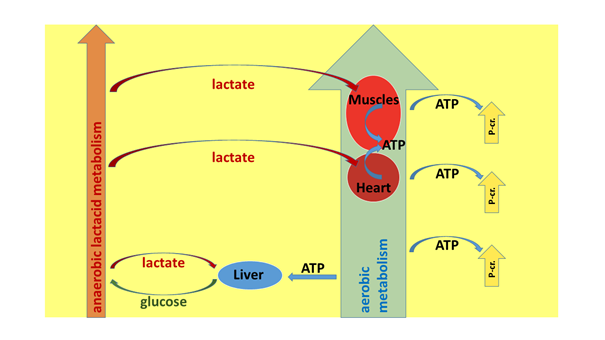
Figure 8: The connections between the systems of energy production
So, what takes place on the system level, is nothing other than that the large-capacity and versatilely trainable aerobic metabolic pathway, as a basis for energy production, disposes of two accessory energy sources, which, however, are continuously generating debt: the anaerobic lactacid source, operating in the longer run, and the anaerobic alactacid one, operating in the short run. Both of these ways of generating extra energy significantly increase the output of performance by muscles, but they do so at the expense of the ATP production of the aerobic system. The relationship between the aerobic and the two anaerobic systems could also be described by imagining the aerobic system as the internal combustion engine of a car, and the anaerobic units as two, performance-augmenting extra batteries. When we put these into service, they lose some of their charge, and after a while, they also go flat – and their recharge can be carried out by the internal combustion engine. Thus, the aerobic system constantly works on repaying the debt generated by its accessory batteries, switched on for momentary demands (Figure 9). If one of them – the lactacid system – has generated a debt of such an extent that the body is not able to pay it back, then the activity stops, or it has catastrophic consequences on the body (lactic acidosis; see later). A good example of the functioning of the energy producing systems is given by the powerful periods in the rounds of combat sports or during the playing of ball games, when competitors speed up movements. In these instances, the anaerobic batteries also switch on, besides the aerobic engine, but the former will shut down before more serious fatigue starts, and their stores will be refilled with the help of the aerobic ATP production. Another example of the synergism of aerobic-anaerobic systems is sprinting, where economising on energy is not a consideration for the competitors, but the performance is attainable with one, abruptly focused effort, and afterwards there is time and a way to regenerate the cellular reserves.
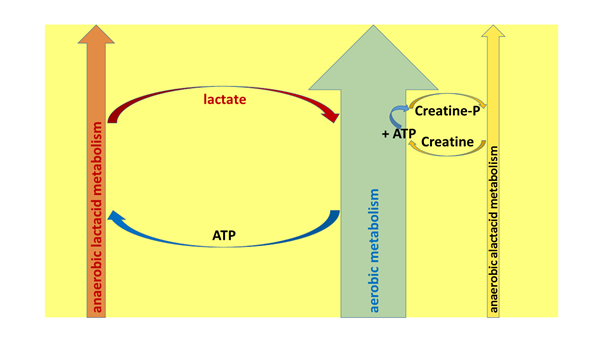
Figure 9: The system-level, essential connections between the aerobic, the anaerobic lactacid and the anaerobic alactacid energy producing systems